
Lithium-sulphur batteries may well be the next generation of power cells that we use in our electric vehicles — if scientists can get them to last longer.
And that’s exactly what a team of researchers at the University of Michigan has tried to accomplish with the development of a 1,000-cycle battery that could quintuple EV range.
What’s the potential of lithium-sulfur batteries?
Lithium-sulfur (Li-S) batteries have a series of advantages over their lithium-ion (Li-ion) counterparts:
- The theoretical energy density of a lithium-sulfur cell is 2,510Wh/kg, compared to a lithium-ion’s 300Wh/kg. This means that Li-S batteries can store two to five times more energy than Li-ion ones, and in turn, they can last substantially longer on a single charge, delivering bigger range.
- Rather than using costly cobalt, which is vulnerable to fragile global supply chains, they use sulphur, which is cheaper and the ninth most abundant element on Earth.

So what’s the hold-up?
Well, the main problem is that Li-S batteries can’t be recharged enough times before they fail to make them commercially viable.
And you guessed it right, it’s all to do with internal chemistry setbacks.
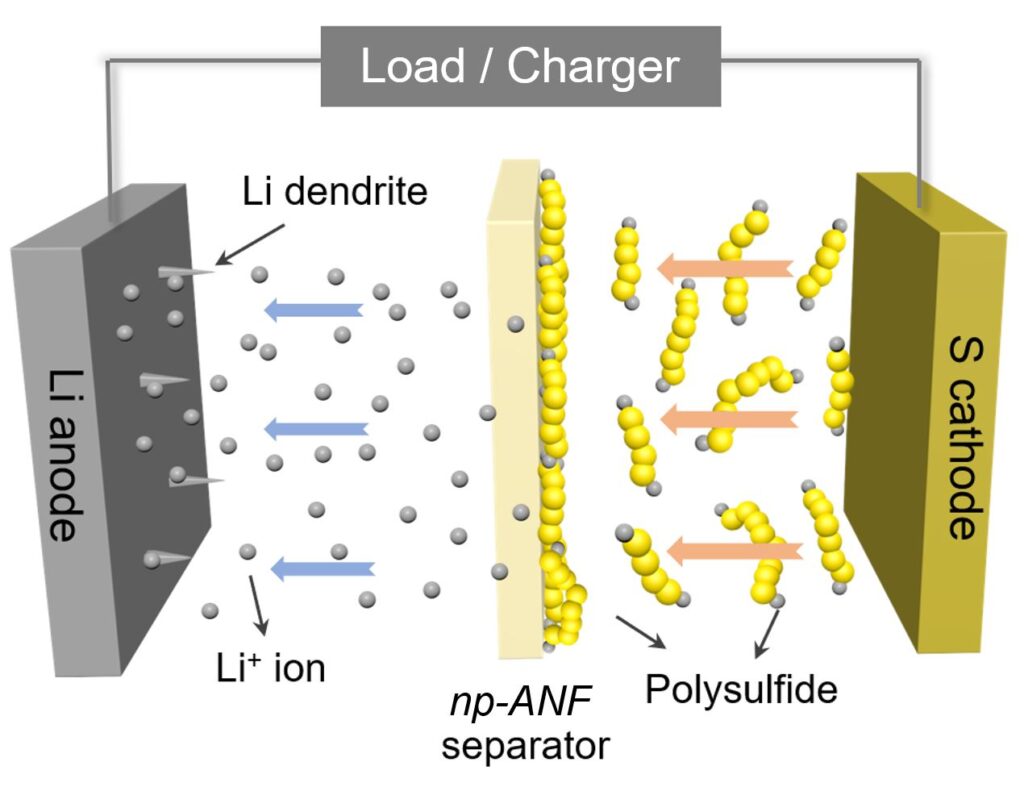
First up, charging a Li-S battery causes a build-up of chemical deposits that degrade the cell and shorten its lifespan.
These deposits form thin, tree-like structures called dendrites, which grow on the lithium anode — the negative electrode inside the battery. As a result, they degrade the anode and the electrolyte, and can potentially cause a short-circuit and overheating.
The second issue here is what’s known as the “lithium polysulfides” problem.
When lithium ions are absorbed by the sulfur electrode (the cathode), they react and form lithium-containing sulfur compounds: the polysulfides.
These compounds not only degrade the suflur cathode, where they grow, but also flow towards the lithium anode and attach themselves to it. This causes the anode’s insulation and degrades the battery’s performance.
One membrane to solve them all
To tackle these problems, the research team developed a bio-inspired membrane made from recycled Kevlar — the same material used in bulletproof vests.
This membrane allowed the researchers to construct a network of aramid nanofibers, which could successfully stop the dendrites’ growth.
But to prevent the flow of lithium polysulfides, the team had to make sure that the membrane had the ability to conduct a process called “ion selectivity.” Simply put, the researchers needed to ensure that Kevlar membrane would allow for the lithium ions to flow between the battery’s anode and cathode, while also blocking the flow of the polysulfides.
To do that, they added an electrical charge to the pores of the membrane and leveraged the polysulfides themselves. As the lithium polysulfides got stuck to the nanofiber membrane, their negative charges repelled the lithium polysulfide ions that continued to form at the sulfur electrode. Positively charged lithium ions, however, could pass freely.
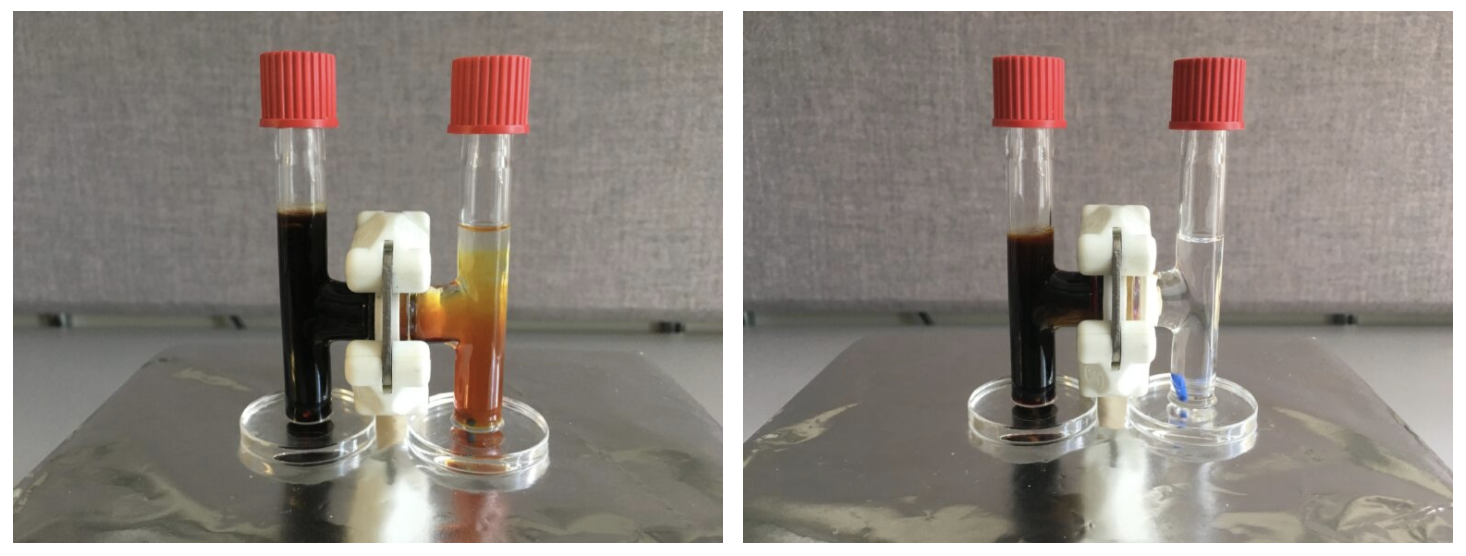
A promising lithium-sulfur battery
According to the lead-researcher Nicholas Kotov, the battery’s design is “nearly perfect,” with its capacity and efficiency approaching the theoretical limits.
What’s more, it can withstand the extreme temperatures of automotive life.
The battery’s real-world cycle life may be shorter with fast charging, approximately 1,000 cycles, he says, which is considered a ten-year lifespan.
The University of Michigan has patented the membrane and Kotov is developing a company to bring it to market. You can find the study paper in Nature Communications.
Get the TNW newsletter
Get the most important tech news in your inbox each week.




